
Current Research in the Girolami Group
Organometallic Chemistry - Can Alkane Complexes be Isolated? We are investigating whether it is possible to prepare kinetically stable coordination complexes in which one of the ligands is an alkane. Although such species have been observed spectroscopically at 10 K and have been surmised to be present as intermediates in certain reactions, none has ever been isolated at or near room temperature. We are investigating the protonation of certain osmium alkyl complexes, whose electronic and steric properties have been chosen so as to favor the formation of an alkane complex. We have found that, in complexes of the type [(C5Me5)L2OsH(CH2R)]+, the hydrogen atoms of the Os-H and Os-CH2R groups are rapidly exchanging even at -100 °C, evidently by means of an alkane intermediate Os(CH3R): Such species offer exciting opportunities to explore the mechanism by which alkane C-H bonds are cleaved by certain organotransition metal species. We are also carrying out related studies of the activation of dihydrogen and organosilanes by transition metals, because the structures of these complexes are closely related to the structures thought to be important in the activation of alkanes.
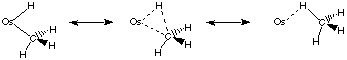 Leading references:
Gross, C. L.; Girolami, G. S. "Metal-Alkane Complexes. Rapid Exchange of Hydrogen Atoms between Hydride and Methyl Ligands in
[(C5Me5)Os(dmpm)(CH3)H+]," J. Am. Chem. Soc. 1998, 120, 6605.
Link to pdf.
Flener, C. E.; Woon, D. E.; Dunning, T. H.; Girolami, G. S. "A DFT and ab initio Benchmarking Study of
Metal-Alkane Interactions and the Activation of Carbon-Hydrogen Bonds," J. Chem. Phys. B. 2010, 114, 1843.
Link to pdf.
Leading references:
Gross, C. L.; Girolami, G. S. "Metal-Alkane Complexes. Rapid Exchange of Hydrogen Atoms between Hydride and Methyl Ligands in
[(C5Me5)Os(dmpm)(CH3)H+]," J. Am. Chem. Soc. 1998, 120, 6605.
Link to pdf.
Flener, C. E.; Woon, D. E.; Dunning, T. H.; Girolami, G. S. "A DFT and ab initio Benchmarking Study of
Metal-Alkane Interactions and the Activation of Carbon-Hydrogen Bonds," J. Chem. Phys. B. 2010, 114, 1843.
Link to pdf.
New Directions in Chemical Vapor Deposition. Chemical vapor deposition (CVD) is an increasingly important technique in industry for the manufacture of integrated circuits and other solid state devices. In CVD, a gas is passed over a hot surface, initiating a chemical reaction in which one of the products is a thin film of a solid such as a metal, semiconductor, or insulator. Most CVD reactions require rather high temperatures (often over 1000 °C), but we are developing new metal organic chemical vapor deposition (MOCVD) precursors and new methods that allow films to be grown at much lower temperatures (below 400 °C). This effort has led us to synthesize some fairly amazing compounds, such as the chromium octahydroborate complexCrB3H8 ,
a completely carbon-free square-planar high-spin chromium(II) complex in which the chromium atom's coordination environment consists of four
hydrogen atoms! This complex serves as an excellent low-temperature CVD precursor to the metallic
ceramic material chromium diboride. Recently, we have been developing new chemical strategies to carry out the deposition of thin
films conformally (i.e, with the same thickness everywhere) and super-conformally (i.e., thicker at the bottom of a recessed feature
than near the top). These capabilities are crucial to the future development of better, faster, and smaller integrated circuits, and
is receiving considerable interest from integrated chip manufacturers. In a related project, we are using CVD and other chemical
approaches to make qbit nanoelectronic devices.
 Leading references:
Mohimi, E.; Zhang, Z.; Mallek, J. L.; Liu, S.; Trinh, B. B.; Shetty, P.; Girolami, G. S.; Abelson, J. R. "Low Temperature CVD of Superconducting Vanadium Nitride
Thin Films," J. Vac. Sci. Technol. A 2019, 37, 031509. Link to pdf.
Leading references:
Mohimi, E.; Zhang, Z.; Mallek, J. L.; Liu, S.; Trinh, B. B.; Shetty, P.; Girolami, G. S.; Abelson, J. R. "Low Temperature CVD of Superconducting Vanadium Nitride
Thin Films," J. Vac. Sci. Technol. A 2019, 37, 031509. Link to pdf.
Abelson, J. R.; Girolami, G. S. "New Strategies for Conformal, Superconformal, and Ultrasmooth Films by Low Temperature Chemical Vapor Deposition," J. Vac. Sci. Technol. A 2020, 38, 030802. Link to pdf
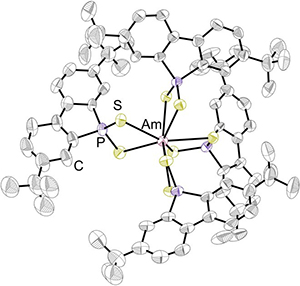
Actinide Chemistry. Nuclear fuel is a clean, carbon-less energy source that for many decades has been helping to meet the world's growing energy demands. However, one primary issue keeping nuclear fuel from realizing its full potential is the accumulation of radioactive waste from nuclear reactors: such waste can be recycled but the current methods to do so suffer from several disadvantages. To address this problem, the Girolami group is investigating the synthesis of new compounds of the actinide elements. This effort involves carrying out studies not only of the chemistry of thorium and uranium, but also that of the trans-uranium elements neptunium, plutonium, and americium. For example, we recently reported what is only the second crystal structure of an americium coordination complex in a non-oxygen ligand environment. We are currently beginning an investigation to synthesize new volatile complexes of the actinide elements in an effort to determine whether actinide elements can be separated from one another by distillation or sublimation. Such an achievement could lead to improved separation methods for nuclear waste recycling.
 Leading references:
Macor, J. A.; Brown, J. L.; Cross, J. N.; Daly, S. R.; Gaunt, A. J.; Girolami, G. S.; Janicke, M. T.; Kozimor, S. A.; Neu, M. P.; Olson, A. C.; Reilly, S. D.;
Scott, B. L. "Coordination Chemistry of 2,2′-Biphenylenedithiophosphinate and Diphenyldithiophosphinate with U, Np, and Pu,"
Dalton Trans. 2015, 18923. Link to pdf.
Leading references:
Macor, J. A.; Brown, J. L.; Cross, J. N.; Daly, S. R.; Gaunt, A. J.; Girolami, G. S.; Janicke, M. T.; Kozimor, S. A.; Neu, M. P.; Olson, A. C.; Reilly, S. D.;
Scott, B. L. "Coordination Chemistry of 2,2′-Biphenylenedithiophosphinate and Diphenyldithiophosphinate with U, Np, and Pu,"
Dalton Trans. 2015, 18923. Link to pdf.
Cross, J. N.; Macor, J. A.; Bertke, J. A.; Ferrier, M. G.; Girolami, G. S.; Kozimor, S. A.; Maassen, J. R. Scott, B. L.; Shuh, D. K.; Stein, B. W. "Comparing the 2,2′-Biphenylenedithiophosphinate Binding of Americium with Neodymium and Europium," Angew. Chem. Intl. Ed. 2016, 41, 12755. Link to pdf.
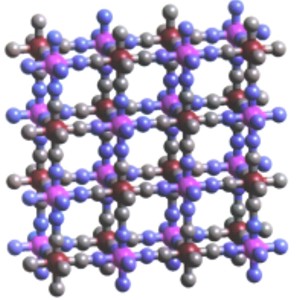
High-TC Molecule-Based Magnetic Materials. We are investigating a building block approach to the synthesis of new magnetic solids. By connecting paramagnetic transition metal coordination complexes into three-dimensional arrays, we are able to make solids that behave as bulk ferro- or ferrimagnets. Our most interesting approach involves preparing metal-substituted analogues of the long-known solid Prussian blue. Prussian blue, which is a cyanoferrate with a cubic unit cell (Fig. 1), becomes magnetic at 5 K. By substituting metal atoms other than iron (particularly vanadium and chromium) into the structure, we can control the magnetic ordering temperature, coercive field, and optical response of the magnetic solid. Whereas molecule-based magnets with magnetic ordering temperatures above -170 °C were unknown when we began our work, we now can prepare crystalline molecule-based magnets with ordering temperatures above +100 °C. The optical properties are of particular interest; for example, it should prove possible to prepare a solid that switches from a diamagnet to a ferromagnet simply by irradiation with light. Such solids may be crucial to the development of computers in the 21st century that use light instead of electrons to carry out computations.
 Leading references:
Holmes, S. M.; Girolami, G. S. "Sol-Gel Synthesis of KVII[CrIII(CN)6]•2H2O:
a Crystalline Molecule-Based Magnet with a Magnetic Ordering Temperature above 100 °C,"
J. Am. Chem. Soc. 1999, 121, 5593. Link to pdf.
Verdaguer, M.; Girolami, G. S. "Magnetic Prussian Blue Analogs," in Magnetism Molecules to Materials V, 2005, pp 283-346.
Link to pdf.
Leading references:
Holmes, S. M.; Girolami, G. S. "Sol-Gel Synthesis of KVII[CrIII(CN)6]•2H2O:
a Crystalline Molecule-Based Magnet with a Magnetic Ordering Temperature above 100 °C,"
J. Am. Chem. Soc. 1999, 121, 5593. Link to pdf.
Verdaguer, M.; Girolami, G. S. "Magnetic Prussian Blue Analogs," in Magnetism Molecules to Materials V, 2005, pp 283-346.
Link to pdf.
Girolami Research Interests.
We are primarily interested in the synthesis, properties, and reactivity of new inorganic, organometallic, and solid state species. Much of the research in our group relates to one of four areas: mechanistic studies of organometallic reactions such as the activation of saturated alkanes, the chemical vapor deposition of thin films from "designed" molecular precursors, the chemistry of the actinides, and the synthesis of new "molecule-based" magnetic materials.Organometallic Chemistry - Can Alkane Complexes be Isolated? We are investigating whether it is possible to prepare kinetically stable coordination complexes in which one of the ligands is an alkane. Although such species have been observed spectroscopically at 10 K and have been surmised to be present as intermediates in certain reactions, none has ever been isolated at or near room temperature. We are investigating the protonation of certain osmium alkyl complexes, whose electronic and steric properties have been chosen so as to favor the formation of an alkane complex. We have found that, in complexes of the type [(C5Me5)L2OsH(CH2R)]+, the hydrogen atoms of the Os-H and Os-CH2R groups are rapidly exchanging even at -100 °C, evidently by means of an alkane intermediate Os(CH3R): Such species offer exciting opportunities to explore the mechanism by which alkane C-H bonds are cleaved by certain organotransition metal species. We are also carrying out related studies of the activation of dihydrogen and organosilanes by transition metals, because the structures of these complexes are closely related to the structures thought to be important in the activation of alkanes.
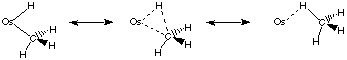
New Directions in Chemical Vapor Deposition. Chemical vapor deposition (CVD) is an increasingly important technique in industry for the manufacture of integrated circuits and other solid state devices. In CVD, a gas is passed over a hot surface, initiating a chemical reaction in which one of the products is a thin film of a solid such as a metal, semiconductor, or insulator. Most CVD reactions require rather high temperatures (often over 1000 °C), but we are developing new metal organic chemical vapor deposition (MOCVD) precursors and new methods that allow films to be grown at much lower temperatures (below 400 °C). This effort has led us to synthesize some fairly amazing compounds, such as the chromium octahydroborate complex
 Leading references:
Mohimi, E.; Zhang, Z.; Mallek, J. L.; Liu, S.; Trinh, B. B.; Shetty, P.; Girolami, G. S.; Abelson, J. R. "Low Temperature CVD of Superconducting Vanadium Nitride
Thin Films," J. Vac. Sci. Technol. A 2019, 37, 031509. Link to pdf.
Leading references:
Mohimi, E.; Zhang, Z.; Mallek, J. L.; Liu, S.; Trinh, B. B.; Shetty, P.; Girolami, G. S.; Abelson, J. R. "Low Temperature CVD of Superconducting Vanadium Nitride
Thin Films," J. Vac. Sci. Technol. A 2019, 37, 031509. Link to pdf.Abelson, J. R.; Girolami, G. S. "New Strategies for Conformal, Superconformal, and Ultrasmooth Films by Low Temperature Chemical Vapor Deposition," J. Vac. Sci. Technol. A 2020, 38, 030802. Link to pdf
Actinide Chemistry. Nuclear fuel is a clean, carbon-less energy source that for many decades has been helping to meet the world's growing energy demands. However, one primary issue keeping nuclear fuel from realizing its full potential is the accumulation of radioactive waste from nuclear reactors: such waste can be recycled but the current methods to do so suffer from several disadvantages. To address this problem, the Girolami group is investigating the synthesis of new compounds of the actinide elements. This effort involves carrying out studies not only of the chemistry of thorium and uranium, but also that of the trans-uranium elements neptunium, plutonium, and americium. For example, we recently reported what is only the second crystal structure of an americium coordination complex in a non-oxygen ligand environment. We are currently beginning an investigation to synthesize new volatile complexes of the actinide elements in an effort to determine whether actinide elements can be separated from one another by distillation or sublimation. Such an achievement could lead to improved separation methods for nuclear waste recycling.

Cross, J. N.; Macor, J. A.; Bertke, J. A.; Ferrier, M. G.; Girolami, G. S.; Kozimor, S. A.; Maassen, J. R. Scott, B. L.; Shuh, D. K.; Stein, B. W. "Comparing the 2,2′-Biphenylenedithiophosphinate Binding of Americium with Neodymium and Europium," Angew. Chem. Intl. Ed. 2016, 41, 12755. Link to pdf.
High-TC Molecule-Based Magnetic Materials. We are investigating a building block approach to the synthesis of new magnetic solids. By connecting paramagnetic transition metal coordination complexes into three-dimensional arrays, we are able to make solids that behave as bulk ferro- or ferrimagnets. Our most interesting approach involves preparing metal-substituted analogues of the long-known solid Prussian blue. Prussian blue, which is a cyanoferrate with a cubic unit cell (Fig. 1), becomes magnetic at 5 K. By substituting metal atoms other than iron (particularly vanadium and chromium) into the structure, we can control the magnetic ordering temperature, coercive field, and optical response of the magnetic solid. Whereas molecule-based magnets with magnetic ordering temperatures above -170 °C were unknown when we began our work, we now can prepare crystalline molecule-based magnets with ordering temperatures above +100 °C. The optical properties are of particular interest; for example, it should prove possible to prepare a solid that switches from a diamagnet to a ferromagnet simply by irradiation with light. Such solids may be crucial to the development of computers in the 21st century that use light instead of electrons to carry out computations.
