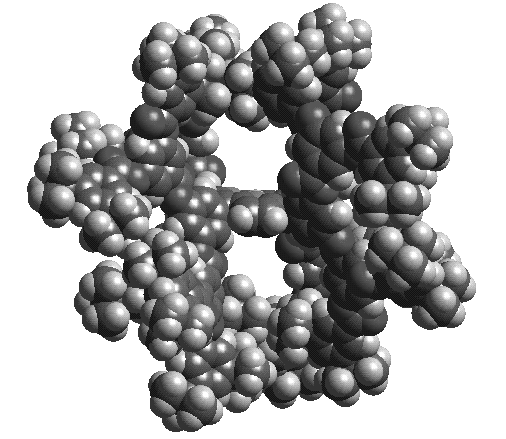EXECUTIVE SUMMARY:PORPHYRIN AND METALLOPORPHYRIN CHEMISTRY
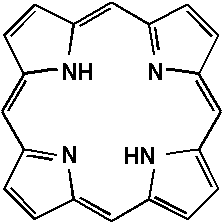
The research team led by Professor Suslick has been at the leading edge of research on synthetic metalloporphyrins for applications as synthetic analogs of heme proteins, shape selective oxidations, and diverse materials chemistry applications including nanoporous network solids, non-linear optical materials, and selective sensors. Background —Porphyrins (which comes from the Greek for "purple") are based on 16-atom rings containing four nitrogen atoms (Fig. 1); they are of perfect size to bind nearly all metal ions. Heme proteins (which contain iron porphyrins) are ubiquitous in nature and serve many roles, including O2 storage and transport (myoglobin and hemoglobin), electron transport (cytochromes b and c), and O2 activation and utilization (cytochrome P450 and cytochrome oxidase). Related macrocycles include the chlorophylls (which have a central magnesium ion) and pheophytins (which are metal free) in the photosynthetic apparatus of plants and bacteria and vitamin B-12 (which has cobalt) found in bacteria and animals.
Fig. 1. Porphine, the simplest porphyrin. In order to gain new insights into the structure and function of metalloproteins, we are involved in the design, synthesis, and physical characterization of complex molecules that look and behave like heme protein active sites. Here we are concerned with issues of oxidation chemistry, catalysis, and molecular recognition [1]. 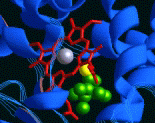
Fig. 2. The active site of cytochrome P450, the heme protein responsible for making water soluble molecules out of hydrophobic ones. Metalloporphyrins and related macrocycles also provide an extremely versatile synthetic base on which to design physical and chemical properties. Another goal of our research effort has been the exploration of metalloporphyrin assemblies as field responsive materials, particularly for photoresponsive applications. Porphyrins can be molecularly engineered to provide erect desirable molecular and materials properties, including very large dipole moments, polarizabilities, and hyperpolarizabilities. The non-linear optical properties of these materials are of special interest. These molecules can be built for energy transfer with molecular control, giving them potential applications in optical communications, data storage, and electrooptical signal processing, as well as in modeling of the photosynthetic reaction center [2]. Recent Accomplishments — To probe the origins of molecular recognition, we have used sterically hindered, "bis-pocket" porphyrins to reverse the normal reactivity of hydrocarbons, enabling us to oxidize selectively primary C-H bonds or the least substituted double bonds. In this work with synthetic metalloporphyrins and with dendrimer-porphyrins, we have synthesized superstructured macrocycles as shape, size, and polarity selective oxidation catalysts for both hydroxylation and epoxidation [1, 3].
Fig. 3. A bis-pocketed dendrimer porphyrin. The protecting pockets on both sides of the porphyrin provides for molecular recognition of incoming substrates and for shape selective catalytic oxidation of alkanes and alkenes. In other work, we are interested in the interaction of metalloporphyrins with peptides [4]. For example, we have designed synthetic heme-peptide complexes by the coordination of amphiphilic peptides to heme and have been examined to determine the influence of the peptide on the properties of the heme and vice versa. The presence of hydrophobic residues flanking a coordinated histidine dramatically increases peptide binding to the heme by a factor of nearly 6000 relative to histidine. Hydrophobic interactions between the porphyrin face and the non-polar side chains of the amino acid residues make a major contribution to the stability of the heme-peptide complexes. Circular dichroism spectra demonstrate that heme binding induces substantial helix formation, presumably to maximize the hydrophobic stabilization. The complexes with the most hydrophobic peptides are the most difficult to reduce, as expected from relative ligand binding to FeII vs. FeIII. Other projects are more materials and biomaterials oriented. For example, we are developing the use of porphyrins as non-linear optical materials and as nanoporous catalytic materials. As one example, we have recently created a new class of bis-pocketed discotic liquid crystals of porphyrins, metalloporphyrins and ligated metalloporphyrins to provide nanostructured assemblies of photoresponsive materials [5].
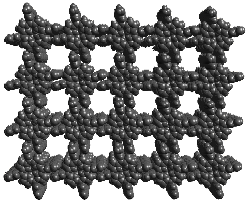
Fig. 4 Bis-pocketed columnar hexagonal liquid crystal porphyrins: n-alkyl esters of 5,10,15,20-(3,5-dicarboxyphenyl)porphyrin, H2DCarPP, R = n-CnH2n+1, n = 8, 10, 12, 14, 16, 18, 20, 22. We are also developing new nanoporous network solids of highly functionalized porphyrins to provide controlled porphyrin orientation in the solid state and to produce molecularly-designed molecular sieves [6]. We intend to explore the use of these solids as heterogeneous shape-selective oxidation catalysts. An example of one such structure is shown below:
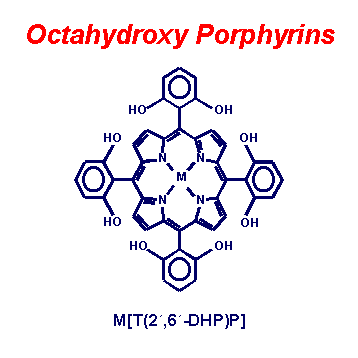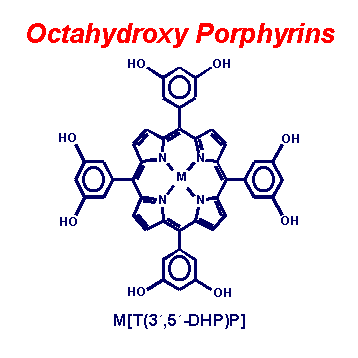
Fig. 5. A pair of octahydroxy substituted porphyrins. Hydrogen bonding networks of these porphyrins are easily prepared in a wide variety of crystal morphologies depending on metal, solvates, and axial ligation.(3,5-dihydroxyphenyl)porphyrin.
Fig. 6. A porphyrin nanoporous solid. Eight hydrogen bonds per porphyrin produce a columnar stacking in this molecular packing diagram of the x-ray structure of tetrakis(3,5-dihydroxyphenyl)porphyrin. Our most recent success has involved the use of metalloporphyrin arrays as sensors for a new concept in artificial olfaction. This electronic nose is based on visualizing the color changes associated with the interaction of vapors with metalloporphyrin dyes. We call this technique "Smell-Seeing" [7].
|
|