The wiring of the nervous system relies on the interplay of intrinsic and extrinsic signaling molecules that control neurite extension, neuronal polarity, process maturation, and experience-dependent refinement. This multigroup effort studies neural circuits in highly controlled microenvironments in order to resolve mechanisms of neuronal development and repair. We engineer multifunctional microfliudic devices of poly(dimethylsiloxane) PDMS to pattern substrate gradients that guide neuron development and compartmentalize neurites into discrete microdomains. One goal is to understand the instructive nature of these gradients for cultured postnatal, primary hippocampal neurons from rat by evaluating directed neuronal polarity. We are extending these approaches by using combinations of bioactive substrates such as laminin and Semaphorin-3A. One aim is to understand how to control neuronal development through these engineered concentration gradients of surface-bound substrates. Our devices allow us to guide neurite migration, to form new neuronal connections, as well as to collect releasates and introduce them to our mass spectrometers to identify the released compounds. |
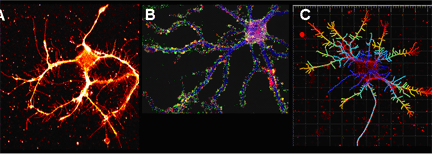
Above: Hippocampal neurons exhibit complex filopodial arborization on poly-L-lysine substrates. A. Confocal image of an immature neuron maintained in culture for 10 days. Collateral filopodia are easily identified by labeling the cell membrane with CM-DiI. B. Mature hippocampal neuron cultured for 33 days labeled immunocyto-chemically with antibodies for MAP2 (blue), synapsin (green), and actin filaments were labeled with rhodamine phalloidin (red). Abundant synaptic terminals and spines are evident in this isolated neuron. C. Analysis of dendritic filopodia formation. Number, length, branchpoints, terminals and volume can be determined for neuronal processes. Scale bars = 20 μm, grid = 10 μm. |
Relevant Publications: J.N. Hanson, M.J. Motola, M.L. Heien, J.W. Mitchell, M.U. Gillette, J.V. Sweedler, R.G. Nuzzo, Textural guidance cues for controlling process outgrowth of mammalian neurons, Lab Chip 9, 2008, 122–131. |