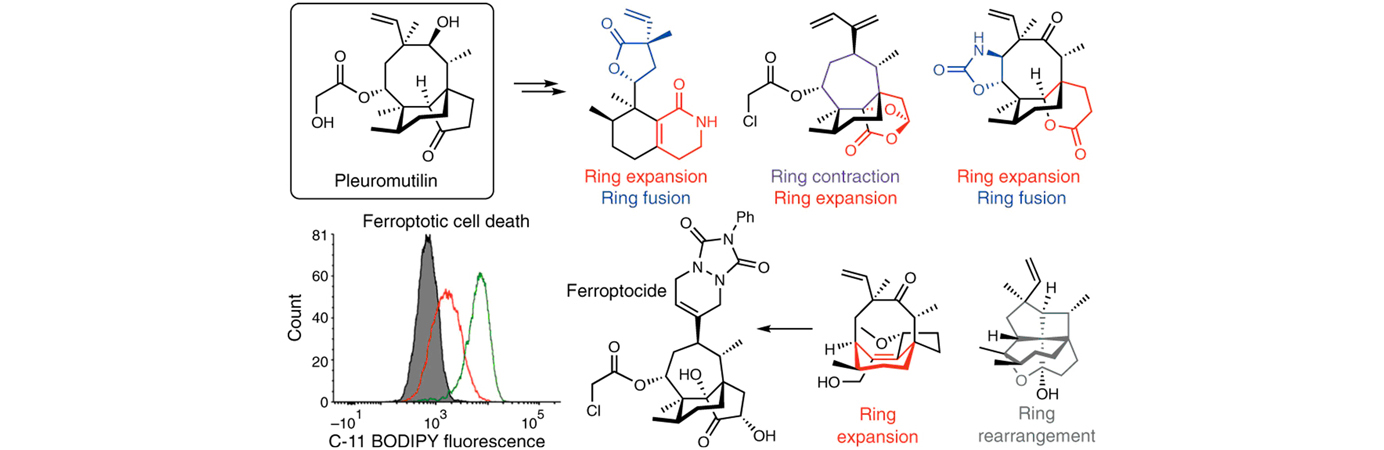
Welcome to the Hergenrother Lab!
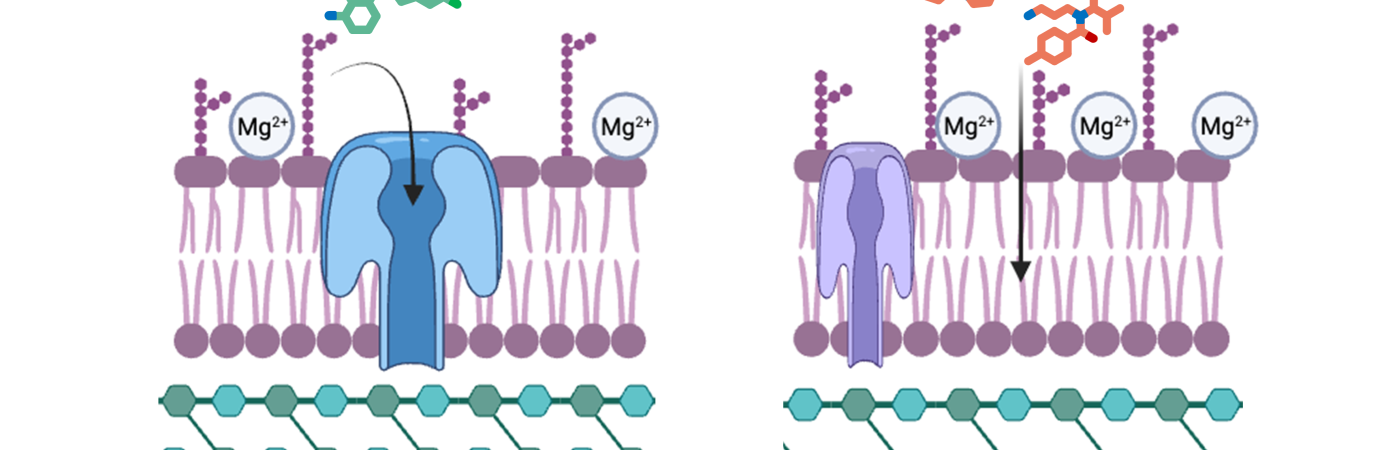
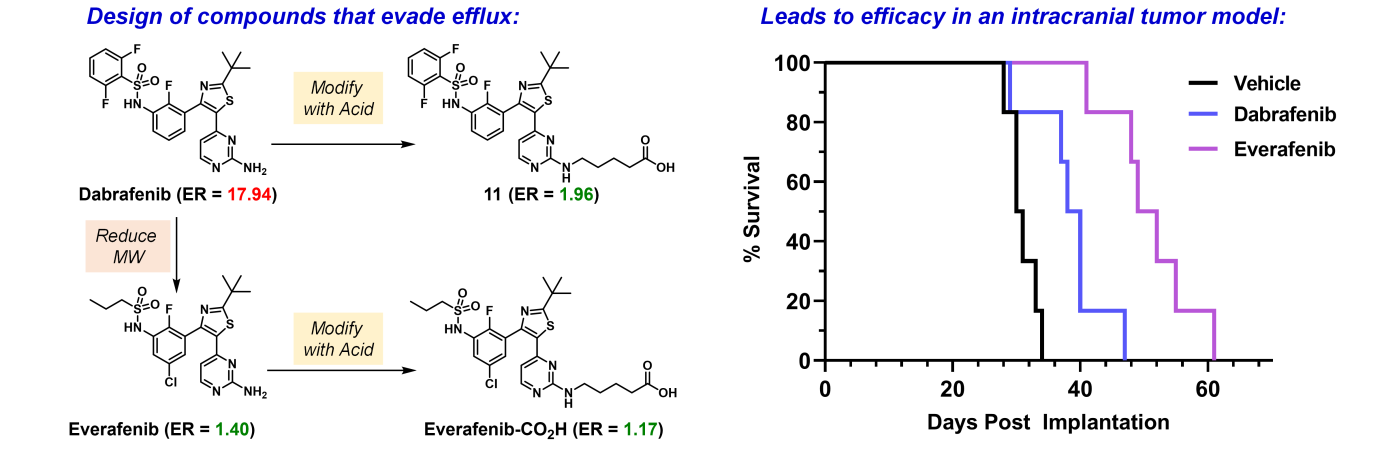
With a guiding theme of using chemistry and small-molecules to identify, explore, and define novel targets for the treatment of disease, we aspire to make an impact in both chemistry and biology. We are both chemists and biologists, using the scientific techniques necessary to solve complex disease-driven questions.
In the Hergenrother lab, we are fully committed to fostering a culture of diversity, inclusion, and equity in our lab and department. Our group welcomes all people with talent of all racial, cultural, and socioeconomic backgrounds, sexual orientations, and gender expressions to join us in our pursuit of scientific discovery. We pledge to be supportive of all individuals and maintain a collaborative and inclusive environment.
Latest News
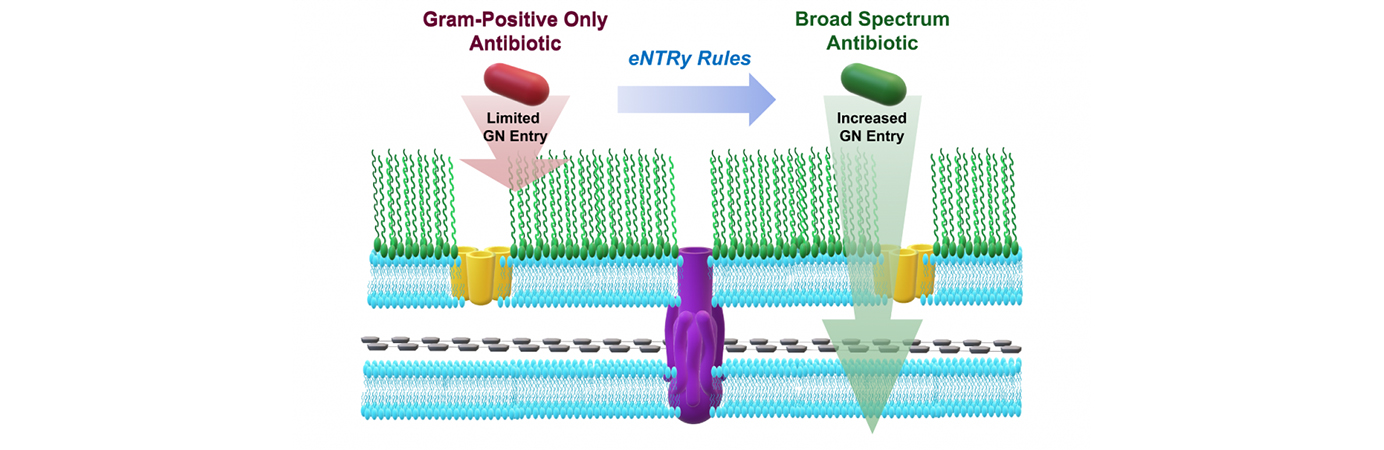
- NEW: Rules for compound accumulation in P. aeruginosa published in Nature.
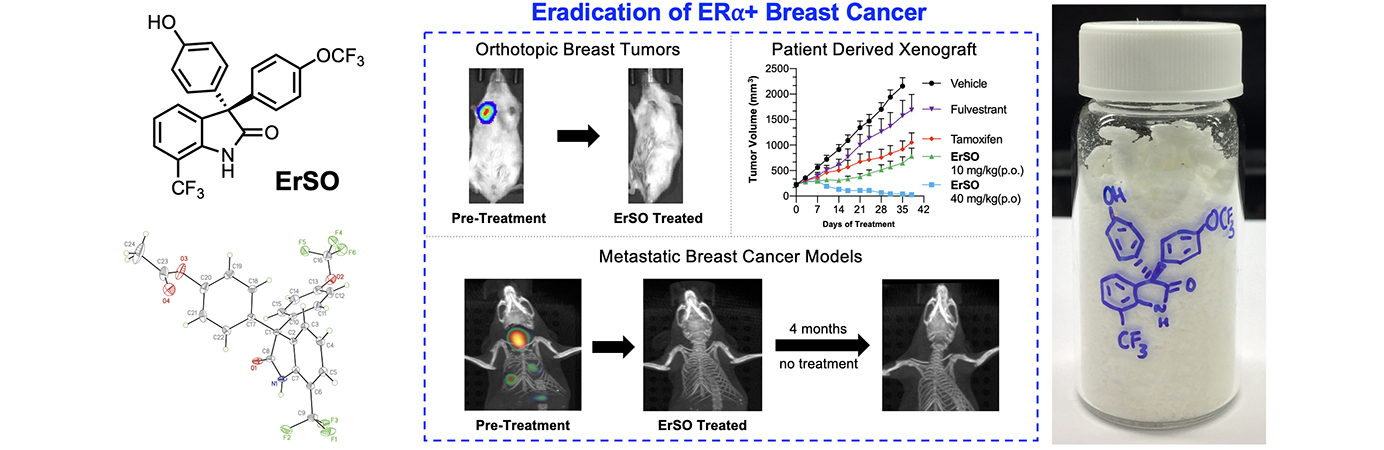
- NEW: A compound from the Hergenrother/Shapiro collaboration has been licensed and is advancing toward treatment of breast cancer.
- Watch the Big Ten Network documentary on the UIUC COVID saliva test, featuring Dr. Diana Ranoa!
- Postdoctoral Positions Open in the Hergenrother Lab
- Congratulations to former student Dr. Matthew Boudreau for winning the Scarborough Award for Graduate/Post-Graduate Excellence from the ACS!