EXECUTIVE SUMMARY:THE CHEMICAL AND PHYSICAL EFFECTS OF ULTRASOUNDby Kenneth S. Suslick
The research team led by Professor Suslick has pioneered the exploration of ultrasound as a tool for chemists. He has developed new applications of sonochemistry in organometallic, inorganic, materials, and biological chemistry. His research at the UIUC has developed new approaches to amorphous and nanostructured materials, has shown great promise for the activation of heterogeneous catalysts, and has created a whole new class of medically important biomaterials. Background — The chemical effects of ultrasound do not come from a direct interaction with molecular species. Instead, sonochemistry and sonoluminescence arises from acoustic cavitation: the formation, growth, and implosive collapse of bubbles in a liquid. Cavitational collapse produces intense local heating (~5000 K), high pressures (~1000 atm), and enormous heating and cooling rates (>1010 K/sec). Acoustic cavitation provides a unique interaction of energy and matter, and ultrasonic irradiation of liquids causes high energy chemical reactions to occur, often accompanied by the emission of light [1].
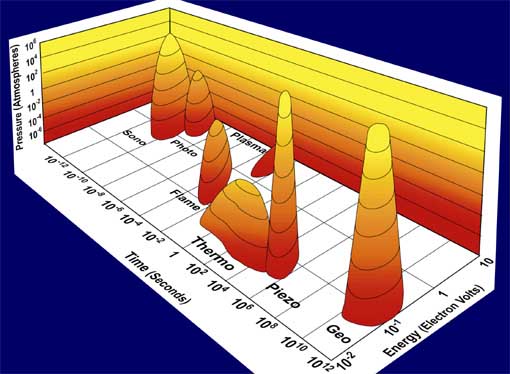
Fig. 1. Acoustic Cavitation: the formation, growth, and implosive collapse of bubbles in a liquid irradiated with high intensity ultrasound. The field of sonochemistry has undergone a renaissance during the past few years, but still remains in its infancy. As our understanding of the nature of the chemical effects of ultrasound has grown, so too has the impact of sonochemistry on the chemical community and on a wide range of the physical sciences. The collapse of bubbles caused by cavitation produces intense local heating and high pressures, with very short lifetimes. Sonoluminescence (Fig. 2) has provided us with a spectroscopic probe of cavitation and permitted determination of the effective temperatures and pressures inside the collapsing bubbles. In clouds of cavitating bubbles, these hot-spots [2] have equivalent temperatures of roughly 5000 K, pressures of about 1000 atmospheres, and heating and cooling rates above 1010 K/s. Thus, cavitation can create extraordinary physical and chemical conditions in otherwise cold liquids. Fig. 3 places sonochemistry in relationship to other forms of chemistry.
Fig. 2. Sonoluminescence from a high intensity ultrasonic horn.
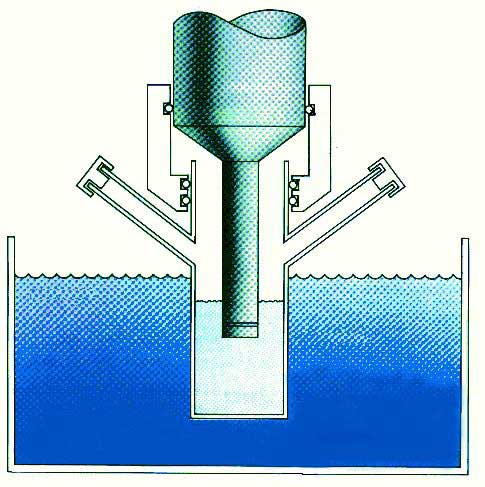
Fig. 3. Chemistry: the interaction of energy and matter. Recent Accomplishments — Ultrasound has proved extremely useful in the synthesis of a wide range of nanostructured materials, including high surface area transition metals, alloys, carbides, oxides and colloids [1]. Sonochemical decomposition of volatile organometallic precursors in high boiling solvents produces nanostructured materials in various forms with high catalytic activities. Fig. 4 shows a typical laboratory apparatus for carrying out sonochemical reactions. Nanometer colloids, nanoporous high surface area aggregates, and nanostructured oxide supported catalysts can all be prepared by this general route. As one example, the recent discovery of a simple sonochemical synthesis of amorphous iron (Fig. 5) helped settle the longstanding controversy over its magnetic properties.
Fig. 4. Typical laboratory apparatus for carrying out sonochemistry.
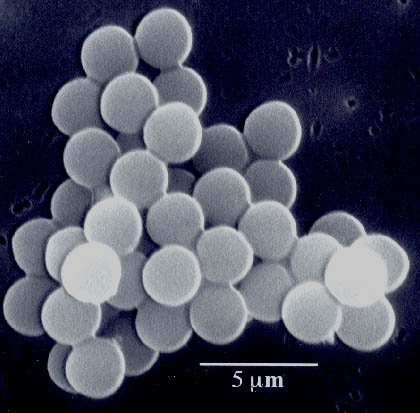
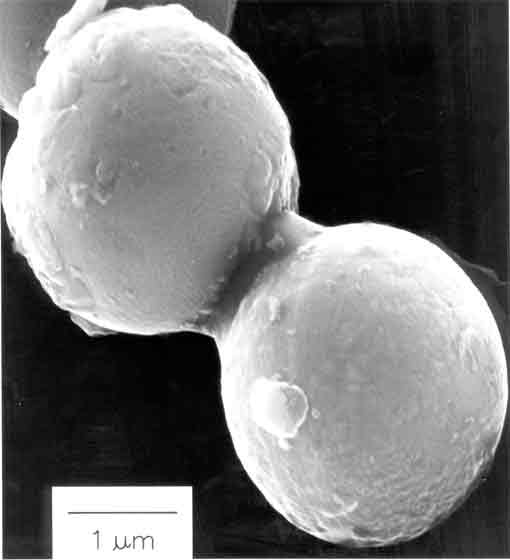
Fig. 5. Scanning electron micrograph of amorphous nanostructured iron powder produced from the ultrasonic irradiation of Fe(CO)5. Particles making up this porous agglomerate are 10 to 20 nm in diameter. Another important application has been the sonochemical preparation of biomaterials, most notably protein microspheres [3]. Using high intensity ultrasound and simple protein solutions, a remarkably easy method to make both air-filled microbubbles and nonaqueous liquid-filled microcapsules has been developed. Fig. 6 shows an electron micrograph of sonochemically prepared microspheres. These microspheres are stable for months, and being slightly smaller than erythrocytes, can be intravenously injected to pass unimpeded through the circulatory system. These protein microspheres, have a wide range of biomedical applications, including their use as echo contrast agents for sonography, magnetic resonance imaging contrast enhancement, drug delivery, among others.
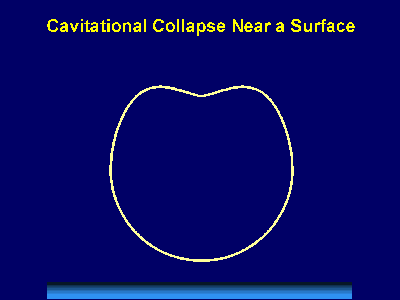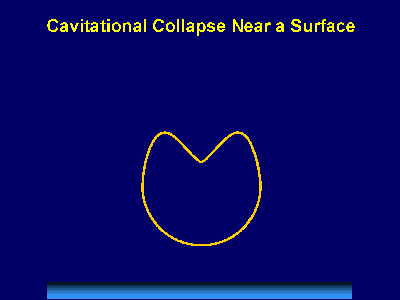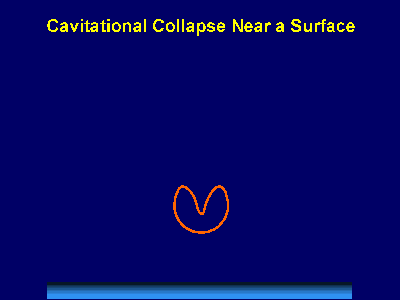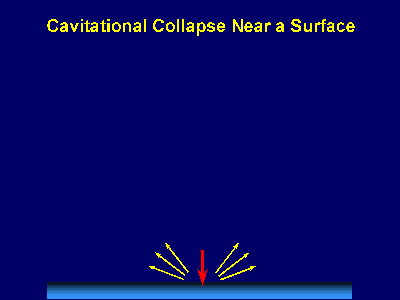
Fig. 6. Scanning electron micrograph of sonochemically synthesized hemoglobin microspheres. When liquids that contains solids are irradiated with ultrasound, related phenomena can occur [1]. When cavitation occurs near an extended solid surface, cavity collapse is non-spherical and drives high-speed jets of liquid into the surface. These jets and associated shock waves can cause substantial surface damage and expose fresh, highly heated surfaces (Fig. 7).
Fig. 7. Bubble collapse near an extended surface is distorted and leads to a shaped-charge effect with liquid jet impacts at >100m/s. Ultrasonic irradiation of liquid-powder suspensions produces another effect: high velocity inter-particle collisions [4]. Cavitation and the shockwaves it creates in a slurry can accelerate solid particles to high velocities (Fig. 8). The resultant collisions are capable of inducing dramatic changes in surface morphology, composition, and reactivity. Heterogeneous catalysts often require rare and expensive metals. The use of ultrasound offers some hope of activating less reactive, but also less costly, metals. For example, the effects of ultrasound on Ni powder is shown in Fig. 9, with the chemical consequence of enormously increasing the catalytic rates of hydrogenation by Ni powder (>105-fold) [5].
Fig. 8. Scanning electron micrograph of 5 m m diameter Zn powder. Neck formation from localized melting is caused by high-velocity interparticle collisions.
Fig. 9. The effect of ultrasonic irradiation on the surface morphology and particle size of Ni powder. Upper image is before ultrasound and lower is after irradiation of a slurry in decane. High-velocity interparticle collisions caused by ultrasonic irradiation of slurries are responsible for the smoothing and removal of passivating oxide coating.
[1](a) K.S. Suslick in Kirk-Othmer Encyclopedia of Chemical Technology; 4th Ed. J. Wiley & Sons: New York, 1998, vol. 26, 517-541. [3] K.S. Suslick, M.W. Grinstaff, J. Am. Chem. Soc. 112, 7807 (1990) [4] S.J. Doktycz, K.S. Suslick Science, 247, 1067 (1990). [5] K.S. Suslick in Handbook of Heterogeneous Catalysis; Ertl, G.; Knozinger, H.; Weitkamp, J.; eds.; Wiley-VCH: Weinheim, 1997; vol. 3, ch. 8.6.
|
|